Sulfur impregnated activated carbon, as a kind of highly efficient mercury removal adsorbent material, has been widely used in various mercury pollution treatment scenarios due to its excellent adsorption performance and environmental adaptability. It effectively makes up for the weak adsorption capacity and poor adaptability of traditional mercury removal materials, and has become one of the core materials in industrial mercury pollution treatment. In this paper, we will comprehensively analyse the application value and development trend of sulfur-carrying activated carbon in the treatment of mercury pollution from the definition of sulfur-carrying activated carbon, mercury-removing mechanism, application scenarios, core advantages, etc., so as to provide references for the treatment of mercury pollution in related industries.
Mercury (Hg), as a heavy metal with strong toxicity, persistence and bioaccumulation, its pollution has become a global environmental hotspot of concern. Mercury pollution mainly comes from coal-fired power plants, natural gas industry, metal smelting and other industrial processes, as well as the production and use of mercury-containing products. These mercury and its compounds will enter the natural environment through atmospheric deposition, wastewater discharge, etc., and eventually pose a serious threat to human health after being enriched step by step through the food chain.
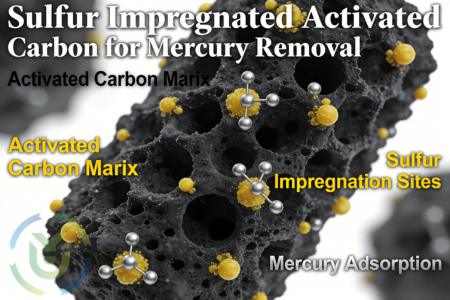
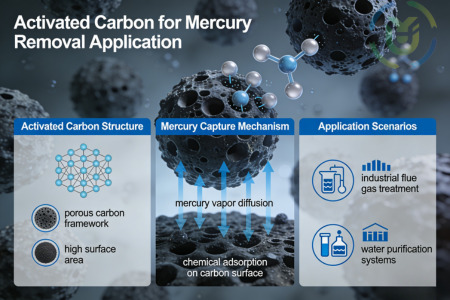
Sulfur impregnated activated carbon is a modified activated carbon prepared by impregnating sulfur-containing compounds (e.g., sulfur) on the surface of ordinary activated carbon, and its core characteristic is to make use of the porous structure of the activated carbon and the chemical activity of sulfur to achieve high efficiency in the adsorption and fixation of mercury. Compared with traditional activated carbon, sulfur-carrying activated carbon has greatly enhanced its affinity for mercury through the modification and optimisation of sulfur, which significantly improves its mercury removal performance and can better meet the needs of treating mercury pollution of different concentrations and forms in industrial scenarios.

By loading elemental sulfur on the surface of activated carbon, sulfur-loaded activated carbon can have a specific chemical reaction with mercury, thus firmly adsorbing and fixing mercury ions, and greatly improving the adsorption capacity and adsorption efficiency for mercury. This specific reaction can form stable mercury compounds and avoid mercury desorption, which completely solves the problems of insufficient adsorption capacity and incomplete adsorption of mercury by traditional activated carbon, and meets the strict requirements for the treatment of industrial-level mercury pollution.
sulfur-carrying mercury-removing activated carbon usually has high mechanical strength, and can maintain stable form during adsorption operation, effectively preventing crushing or breakage. This characteristic can avoid the blockage of the pipeline and filter of the treatment system caused by the pulverisation of the activated carbon, reduce the maintenance cost and downtime loss of the equipment, effectively guarantee the long-term stable operation of the treatment system, and improve the overall treatment efficiency.
This kind of activated carbon can maintain stable chemical performance under different operating conditions, and can play an efficient role in a wide range of pH value and temperature conditions. Whether it is acidic industrial tail gas, alkaline wastewater, or high and low temperature environment, it can stably adsorb mercury pollutants, and adapt to the needs of tail gas and wastewater treatment in different industries, with strong versatility.
Elemental sulphur is firmly fixed on the surface of activated carbon through a special process, which is not a simple physical mixing, so it is not easy for sulphur to leak out during operation. This advantage effectively reduces the possibility of secondary pollution, not only ensures the stability of mercury removal effect, but also avoids the damage caused by sulphur leakage to the surrounding environment and equipment, taking into account the mercury removal effect and environmental safety.
Sulfur-carrying activated carbon contains calcium sulfide, ammonium sulfide, sodium sulfide and other sulfur sources, which are uniformly attached to the surface of the activated carbon and the pores through impregnation process, providing sufficient active sites for mercury reaction. These sulphides can react specifically with gaseous mercury in the flue gas to produce mercury sulphide (HgS). Mercury sulphide is extremely stable in the natural environment, has low volatility and is not easy to be re-released into the atmosphere, thus achieving permanent fixation of mercury and effectively avoiding secondary mercury pollution.
Some of the sulphur-carrying activated carbons are modified with a composite modification process, whereby the element sulphur is loaded with additional chlorinated compounds such as calcium chloride and sodium chloride, in order to increase the capture capacity of the different forms of mercury. Some sulphur-carrying activated carbons are modified with a composite process to load elemental sulphur with additional chlorides such as calcium chloride and sodium chloride to enhance the capture of different mercury forms. If the sulphur-carrying activated carbon is also loaded with such chlorides, the gaseous mercury in the flue gas reacts rapidly with these chlorides to form mercuric chloride (HgCl₂). Mercury chloride no longer exists in gaseous form, but is converted into a stable solid mercury compound, which is easy to be separated and processed subsequently by filtration, sedimentation, etc. The chemical reaction formula is: Hggas + Cl₂ → HgCl₂.
During the preparation of sulphur-loaded activated carbon, a small amount of oxides are naturally formed on the surface, and these oxides work synergistically with elemental sulphur to further enhance the mercury removal effect. The oxides on the surface of the sulphur-containing activated carbon react with gaseous mercury in the flue gas, converting the mercury to mercury oxide (HgO). This process can effectively immobilise mercury molecules, reduce their ability to migrate in the gas, reduce the diffusion of mercury, and further enhance the overall mercury removal efficiency, with the chemical reaction formula: Hggas + O₂ → HgO.
In some of the sulphur-loaded mercury-removing activated carbons, the loaded sulphur source not only reacts with the mercury, but also reacts with the sulphur dioxide (SO₂) in the flue gas to produce sulphate or sulphite ( Such as calcium sulfate CaSO₄, calcium sulfite CaSO₃), in order to achieve the removal of mercury at the same time to complete the desulphurisation, two birds with one stone. The main chemical reaction formula with sulfur dioxide is: SO₂ + Ca(OH)₂ → CaSO₃ + H₂O, SO₂ + 1/2O₂ + CaO → CaSO₄, this reaction can reduce the concentration of mercury and sulfur dioxide in the flue gas at the same time to reduce the harm to the environment of multiple pollutants.

Natural gas usually contains trace amounts of mercury, which are extremely low, but long-term transport in pipelines will cause serious corrosion of metal pipelines, valves and other equipment, shorten the service life of the equipment, and increase the cost of maintenance. At the same time, mercury impurities also affect the quality of natural gas, especially in high-end natural gas application scenarios. Sulfur-carrying activated carbon, with its highly efficient mercury adsorption capacity, can accurately capture trace amounts of mercury in natural gas and completely remove mercury impurities, which not only ensures the safety and stability of natural gas transmission, but also improves the quality of natural gas and meets the needs of various applications.
In the hydrogen production process, whether it is water electrolysis, natural gas reforming or other production processes, traces of mercury impurities may be mixed in, and the presence of mercury will seriously affect the purity of hydrogen, which will not be able to meet the requirements of hydrogen applications in electronics, semiconductors, pharmaceuticals and other high-end fields. In addition, mercury impurities can also damage catalysts and precision components in subsequent production equipment, resulting in reduced productivity and frequent equipment failures. Sulphur-carrying activated carbon enables the deep removal of mercury from hydrogen, keeping the mercury content at a very low level, which not only meets the strict purity requirements of high-end hydrogen applications, but also protects the production equipment and ensures that production runs smoothly.
Industrial production processes produce a large number of mercury-containing waste gases, which are emitted into the air and cause atmospheric mercury pollution, which in turn endangers the ecological environment and human health through deposition and food chain enrichment. Sulfur-carrying activated carbon is suitable for air purification in all kinds of industrial sites, and can also efficiently treat mercury in the flue gas emitted from coal-fired power plants, waste incineration plants, etc. It can quickly capture mercury in the flue gas. It can quickly capture gaseous mercury and mercury compounds in the flue gas, effectively reduce the mercury content in the air, reduce the atmospheric mercury pollution load, and help enterprises to achieve air pollutant emission standards and guard the safety of the ecological environment.
Metal smelting, chemical production, battery manufacturing and other industries will emit a large amount of industrial tail gas containing mercury in the production process, the mercury content in this kind of tail gas is relatively high, and if it is directly emitted, it will seriously pollute the environment, and at the same time, it does not meet the strict requirements of the national environmental protection laws and regulations. Sulfur-carrying activated carbon can capture the mercury and mercury compounds in the tail gas efficiently and reduce the mercury content in the tail gas to below the emission standard. It can not only ensure that the tail gas of enterprises meets the emission standards, avoid the risk of environmental protection penalties, but also reduce the harm of mercury pollution to the surrounding environment and human health.
As an important raw material in chemical and energy fields, syngas is easy to be mixed with mercury impurities during the production process, which will affect the subsequent conversion reaction of syngas, reduce product quality, and even lead to catalyst poisoning and deactivation, affecting the stability of production. Sulfur-carrying activated carbon has excellent mercury adsorption performance and chemical stability, which can adapt to the complex working conditions of syngas and realise the deep purification of mercury in syngas, completely removing mercury impurities. This not only ensures the stable operation of the subsequent production process, but also improves the quality of syngas-derived products and reduces production losses.
Laboratories conducting mercury-related experiments, pharmaceutical production process when the use of mercury-containing raw materials, will produce a small amount of mercury-containing waste gas, such emissions are not large, but the mercury toxicity is extremely strong, if the direct emission will pollute the laboratory and production workshop air, endangering the health of experimental personnel, production personnel. Sulfur-carrying activated carbon can accurately remove mercury from these low concentration mercury-containing exhaust gases with high adsorption efficiency and no secondary pollution. It can create a safe and environmentally friendly experimental and production environment, protect the health of personnel, and meet the environmental management requirements of laboratories and the pharmaceutical industry.
Sulfur-carrying activated carbon can adsorb specific pollutants such as mercury after sulfur-modified treatment, and its affinity for mercury is greatly improved compared with that of unmodified ordinary activated carbon. For low concentration mercury pollution which is difficult to be treated by traditional activated carbon, it can still achieve high efficiency and accurate removal, and the adsorption effect is more targeted, which can meet the strict requirements of low concentration mercury treatment in industrial scenarios.
Compared with ordinary activated carbon, sulfur-carrying activated carbon has a significantly higher mercury adsorption capacity, which can not only efficiently treat high-concentration mercury pollution scenarios, but also cope with the test of complex working conditions. It has strong anti-interference ability and is not easily affected by other impurities in flue gas and wastewater, so it can still maintain stable mercury removal performance under complicated working conditions, ensuring that the treatment effect is stable and meets the standard.
The adsorbent material has strong adaptability and can be widely used in many industries and many scenarios of mercury pollution treatment. Whether it is gaseous mercury, liquid mercury, low concentration or high concentration of mercury pollution, it can play a good mercury removal effect, without the need to customise it for different scenarios, which greatly reduces the cost of treatment and the difficulty of adaptation.
Although the initial purchase cost of sulfur-carrying activated carbon is slightly higher than that of ordinary activated carbon, its cost-effective advantage is very significant in terms of the comprehensive long-term use cost. Due to its large adsorption capacity and long service life, it can reduce the frequency of replacement, and at the same time, it can ensure that the emissions meet the standards and avoid fines due to exceeding the environmental protection standards, so it can achieve obvious cost savings in the long run.
High mercury removal efficiency, medium cost, low operational complexity, no need for complex equipment investment and maintenance, applicable to flue gas, natural gas and other scenarios of mercury treatment, taking into account the efficiency and economy, it is currently one of the most widely used mercury removal technologies.
High mercury removal efficiency, medium cost, low operational complexity, similar performance to sulphur-carrying activated carbon, but more suitable for mercury removal from flue gas in coal-fired power plants, more targeted, and not as well adapted as sulphur-carrying activated carbon in other industries.
Mercury removal efficiency is medium, the cost is high, the equipment operation and maintenance complexity is high, need to invest a lot of equipment and manpower costs, mainly applicable to large-scale mercury pollution control in large industrial plants, small-scale scenarios are less economical.
High mercury removal efficiency, high cost, medium operation complexity, requires professional equipment and maintenance personnel, applicable to some special industries, special working conditions of mercury pollution control, poor versatility, difficult to widely promote the application.
|
Mercury removal technologies |
Applications |
|
Sulfur impregnated Activated Carbon |
Flue gas, natural gas, etc., with a wide range of applications |
|
Halocarbons |
Mercury removal from flue gas of coal-fired power plants |
|
Wet scrubbers |
Large-scale treatment of large industrial plants |
|
Selenium-based adsorbents |
Special industries, special working conditions |
The optimal temperature range of sulfur-carrying activated carbon in removing mercury is 120℃-180℃, and the optimum range is verified by a large number of industrial tests. This range is the optimal range verified by a large number of industrial tests. Within this temperature range, its adsorption performance and chemical reaction activity are the strongest, and the mercury removal efficiency can reach the best level; if the temperature is out of this range, whether it is too high or too low, it will significantly reduce its mercury adsorption capacity, which directly affects the overall mercury removal effect and increases the cost of treatment.
Sulphur dioxide, nitrogen oxides (NOx) and moisture in the flue gas are the main interfering factors affecting the mercury removal efficiency. These substances will compete with mercury for active adsorption sites on the surface of the sulfur-carrying activated carbon, and may change the chemical environment on the surface of the activated carbon, thereby inhibiting the adsorption and reaction of mercury and decreasing the efficiency of mercury removal. Therefore, in complex flue gas scenarios, it is necessary to do a good job of pre-treatment work in order to reduce the interference.
Mercury exists in the form of elemental mercury (Hg⁰) and oxidised mercury (Hg²⁺), both of which have different physico-chemical properties and are difficult to remove. Among them, oxidised mercury is easier to be captured by sulphur-carrying activated carbon, while elemental mercury is relatively more difficult to be removed. Therefore, the proportion of mercury in the flue gas will directly determine the selection of the type of sulphur-carrying activated carbon and the amount of injection, which will affect the treatment effect.
The performance parameters of sulfur-carrying activated carbon itself are the core intrinsic factors that determine its mercury removal efficiency. Its pore structure, specific surface area, as well as the type and amount of sulfur source loaded on the surface will affect its affinity and reaction kinetics with mercury, which in turn will directly affect the efficiency of mercury removal, so it is necessary to select sulfur-carrying activated carbon with appropriate performance parameters according to the actual demand for treatment.
The core of the R&D of sulphur-carrying activated carbon is to achieve performance upgrading through the optimisation of the process, and the industry is currently focusing on the iteration and improvement of the modification process. By optimising the modification process, accurately adjusting key parameters such as sulphur loading and pore structure, as well as combining chlorine, selenium and other functional elements for composite modification, the mercury removal efficiency and service life of sulphur-carrying activated carbon can be further enhanced. This R&D direction can effectively break through the performance bottleneck of the existing products, expand their scope of application under more complex and demanding working conditions, and meet the individual treatment needs of different industries.
At present, the optimal mercury removal temperature of sulfur-carrying activated carbon is 120℃-180℃, and this temperature limitation restricts its application in low-temperature working conditions. In the future, we will focus on the research and development of low-temperature type sulfur-carrying activated carbon, and reduce the optimal mercury removal temperature through the optimisation of the modification process, so that it can be adapted to the treatment of mercury pollution in special scenarios such as low-temperature flue gas and low-temperature wastewater. This technological innovation will not only expand the application scenarios of sulphur-carrying activated carbon, but also reduce energy consumption under low-temperature working conditions and improve the economy and environmental protection of the treatment process.
The future mercury pollution control will get rid of the limitation of single-pollutant control, and develop in the mainstream direction of ‘one machine, multiple control’ and ‘synergistic control’. Sulfur-carrying activated carbon mercury removal technology and the existing desulfurisation, denitrification, dust removal and other pollutant management technology in-depth integration, to build an integrated multi-pollutant control system, can be realised with other pollutants and mercury synchronous management. This integration mode can greatly improve the overall management efficiency, but also reduce the investment in building multiple separate management systems, effectively reducing the cost of corporate pollution management.
As global environmental protection awareness continues to rise, the regulatory requirements for mercury pollution control in various countries are tightening, and emission standards are becoming increasingly stringent. To cope with this trend, sulfur-carrying activated carbon will further optimise its performance to accurately match the environmental emission requirements of different countries and regions, breaking the geographical application restrictions. At the same time, it will be iterated in the direction of green, environmental protection and no secondary pollution, so as to improve the effect of mercury removal while reducing the potential impact on the environment, and to promote the green upgrading of the entire mercury pollution treatment industry.
Sulfur impregnated activated carbon, as an efficient and universal mercury removal adsorbent material, plays a significant role in the treatment of mercury pollution in many industries by virtue of its excellent adsorption performance, environmental adaptability and economy, and achieves efficient fixation of mercury through a variety of chemical reactions, which is of greater value for promotion than other mercury removal technologies.
In practical application, it is necessary to pay attention to the influence of temperature, flue gas composition and other key factors on the efficiency of mercury removal, and to do a good job in the safe disposal of activated carbon after adsorption; in the future, with the relevant technological innovation and integration, the sulfur-carrying activated carbon mercury removal technology will be upgraded continuously, to adapt to the more stringent environmental protection requirements, and to provide better treatment solutions.